Osaka-Gunze Limited (Headquarters: Osaka, Japan, President: Atsushi Hirochi)[TOKYO: 3002] is pleased to announce that a subsidiary “Medical U&A (hereinafter MUA)” has obtained government approval to produce and sell the long-term hair reduction diode laser "MeDioStar®Monolith" in Japan. The company is planning to launch the product in April 2021.
1. About the long-term hair reduction market
The long-term hair reduction is getting popular in Japan. And the laser hair removal device market is expanding accordingly. During laser hair removal, a laser emits a light that is absorbed by the pigment (melanin) in the hair. The light energy is converted to heat, which damages the tube-shaped sacs within the skin (hair follicles) that produce hairs and the hair removal is promoted. In the past, most hair removal has been performed on adult females, but with the diode laser devices that are less painful and gentle on the skin are becoming popular, the users like males, children and care recipients are expanding accordingly.
As the market becomes more widespread, there is a demand for diode lasers that are easier to use, and the long-term hair reduction diode laser "MeDioStar Monolith" to be released this time to meet the demand.
2. Features of “MeDioStar Monolith”
The long-term hair reduction diode laser "MeDioStar Monolith" is a 6th generation diode laser developed by Asclepion Laser Technologies GmbH in Germany.
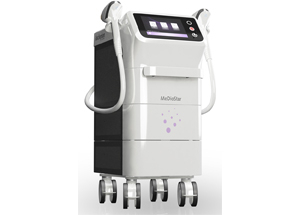
(1) Two-handpiece system
Multiply your treatment options with our new two-handpiece system and save time with multiple handpieces connected simultaneously.
(2) Handpieces with 360° skin cooling
The 360° contact skin cooling system cools the epidermis very efficiently and protects the skin from burns.
(3) Maximum power, mix of wavelengths & dynamic mode
The new features make the treatment more effective, faster and gentler than ever before.
(4) Touchscreen LCD with redesigned software
The product has a touchscreen LCD featuring a completely redeveloped and intuitive user interface.
(5) Wireless footswitch
The new, safe and modern footswitch enables convenient treatment.
3. Product outline
- Product name
- MeDioStar Monolith
- Intended use or effect
- The device is intended to achieve stable long-term hair reduction by selective photothermolysis.
- Head Office
- 5F, Shinfujita Bld., 2-4-27, Dojima, Kita-ku Osaka, Osaka, 530-0003
- Approval number
- 30200BZX00404000
- Manufacture
- Asclepion Laser Technologies GmbH
4. Marketing Strategy
The company strives to sales expansion through educating the new product features.
5. About the Medical U&A
- Established
- April, 1986
- President
- Shojiro Matsuda
- Head Office
- 5F, Shinfujita Bld., 2-4-27, Dojima, Kita-ku Osaka, Osaka, 530-0003
- Main Business
- ・Sales of medical instruments (Plastic and Reconstructive surgery, Neurosurgery, Oral surgery, Cardiovascular surgery, Cosmetic surgery, Pediatric surgery, Dermatology etc.)
・Consultation for opening medical facilities
For further information, please contact:
- Press
- Gunze Limited
Corporate Communication Department
TEL: 81-6-6348-1314 (Shinji Nonaka)
- Visit Homepage
- https://www.gunze.co.jp/english/
